A Sample of Zinc Metal Reacts Completely With
The volume of the gas is 780 L and the pressure is 0980 atm. MathrmZns2 mathrmHCla q longrightarrow mathrmZnCl_2a qmathrmH_2g The hydrogen gas produced is collected over water at 250circ mathrmC using an arrangement similar to that shown in Figure 1014mathrma.
10 Zinc Metal Reacts With Hydrochloric Acid To Form Zinc Chloride And Hydrogen Gas A Write Homeworklib
The water vapor pressure at 220 degrees Celsius is 198 mm Hg and the height of water column in the gas collecting tube is 120 cm the density of mercury is 136.
. A sample of zinc metal reacts completely with an excess of hydrochloric acid. If sodium peroxide is added to water elemental oxygen gas is generated. A sample of zinc metals reacts completely with excess hydrochloric acidZns 2 HClaq ZnCl2aq H2g If 7802 mL of gas is collected over water at 200C and a pressure of 7540mmHg determine the initial mass of zinc used in grams.
The acid quantity of 55 g can react with 654 55 73 493 g of Zn and 057 g Zn remain unreacted. A volume of 1327 mL of the base was. Calculate the amount of zinc metal in grams consumed in the reaction.
The reaction takes place according to Zn 2 HCl ZnCl2 H2. A sample of zinc metal reacts completely with an excess of hydrochloric acid. Zn s 2HCI aq ZnCl2 aq H2 g The hydrogen gas produced is collected over water at 25C using an arrangement similar to that shown in Figure 515.
Zn s 2HCl aq --- ZnCl2 aq H2 g The hydrogen gas produced is collected over water at 250 degrees C. Chemistry Gases Molar Volume of a Gas 1 Answer. Binary compounds of alkali metals and hydrogen react with water to liberate.
Zns2HClaq-ZnCl2aqH2g How many milliliters of 450M HCl1aq are required to react with 825g of an ore containing 300 Zns by mass. MathrmZns2 mathrmHCla q longrightarrow mathrmZnCl_2a qmathrmH_2g. Zinc has an atomic mass of 654 g and the sum of two molecular masses of HCl makes 73 g.
A sample of zinc metal reacts completely with an excess of hydrochloric acid. Zns 2HClaq -- ZnCl2aq H2g The hydrogen gas produced is collected over water at 250 C. Zinc reacts with hydrochloric acid according to the reaction equation.
And the atmospheric pressure is 0980 atm. A sample of a vitamin supplement was analyzed by titrating a 03252 g sample dissolved in water with 00284 M NaOH. What mass of sodium reacted.
The wet pressure of the collected gas was 745 mm of Hg and the volume was 780 Liters. Z n s 2 H C l a q Z n C l 2 a q H 2 g The hydrogen gas produced is collected over water at 250 C using an arrangement similar to that shown in Figure 1114 a The volume of the gas is 780 L and the pressure is 0980 atm. A sample of zinc metal reacts completly with an excess of hydrochloric acid.
Note that the vapor pressure of water at 200C is 17535 mmHg. The volume of the gas is 780 L the pressure is 0980 atm. The volume of the gas is 780 L.
What volume of hydrogen at STP is produced when 25 g of zinc react with an excess of hydrochloric acid in the reaction Zn 2HCl - ZnCl_2 H_2. Zns 2HClaq -- ZnCl2aq H2g The hydrogen gas produced is collected over water at 250 C. A 01358g zinc metal reacts completely with Dil HCL to produce 5220ml of H2g at 220 degrees Celsius.
A sample of zinc metal reacts completely with an excess of hydrochloric acid. Zinc metal reacts with hydrochloric acid according to the following balanced equation. Mathrm Zn s2 mathrm HCl a q longrightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The hydrogen gas produced is collected over water at 250 circ mathrm C using an arrangement similar to that shown in Figure 1114 mathrm a The volume.
Electrons that are produced due to the chemical reaction are transferred from the zinc metal to the copper metal through a conducting wire producing the electric current. 19 A sample of Zinc metal reacts completely with an excess of Hydrochloric Acid as shown in the reaction below. A sample of zinc metal reacts completely with an excess of hydrochloric acid.
A sample of zinc metal reacts completely with an excess of hydrochloric acid 0247. A sample of sodium reacts completely with 0355kg of chlorine forming 585g of sodium chloride. Ascorbic acid vitamin C is a diprotic acid having the formula H2C6H6O6.
The volume of the gas is 780 L the pressure is 0980 atm. Z n s 2 H C l a q Z n C l 2 a q H 2 g beginalign mathrmZns2mathrmHClaqrightarrowmathrmZnCl_2aqmathrmH_2g endalign Zn s 2 HCl a q ZnC l 2 a q H 2 g. Zn 2HClaq ZnCl H2O The hydrogen gas was collected over water at 25C.
General Chemistry 6th Edition Edit edition Solutions for Chapter 5 Problem 64Q. Calculate the amount of zinc metal in grams consumed in the reaction. A sample of zinc metal reacts completely with an excess Zns 2HClaq ZnCl2aq H2g The hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 515.
A sample of zinc metal is allowed to react completely with an excess of hydrochloric acidThe hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 514. SOLVEDA sample of zinc metal reacts completely with an excess of hydrochloric acid. A sample of zinc metal reacts completly with an excess of hydrochloric acid.
Add To Playlist. The hydrogen gas is collected over water at 220 degree Celsius and a barometric pressure of 755 mm Hg. A sample of zinc metal reacts completely with an excess of hydrochloric acid.
So for equal masses of Zn and HCl the metal is în excess. A sample of zinc metal reacts completely with an excess of hydrochloric ZnCl2aq H2g The hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 515.
Solved 5 Zinc Metal Reacts With Hydrochloric Acid To Chegg Com
Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com
Solved Question 1 Zinc Metal Reacts With Aqueous Chegg Com
Pin By Sanghita Dey On Cbse Class 10 In 2021 Solutions Homogeneous Mixture Bullet Journal
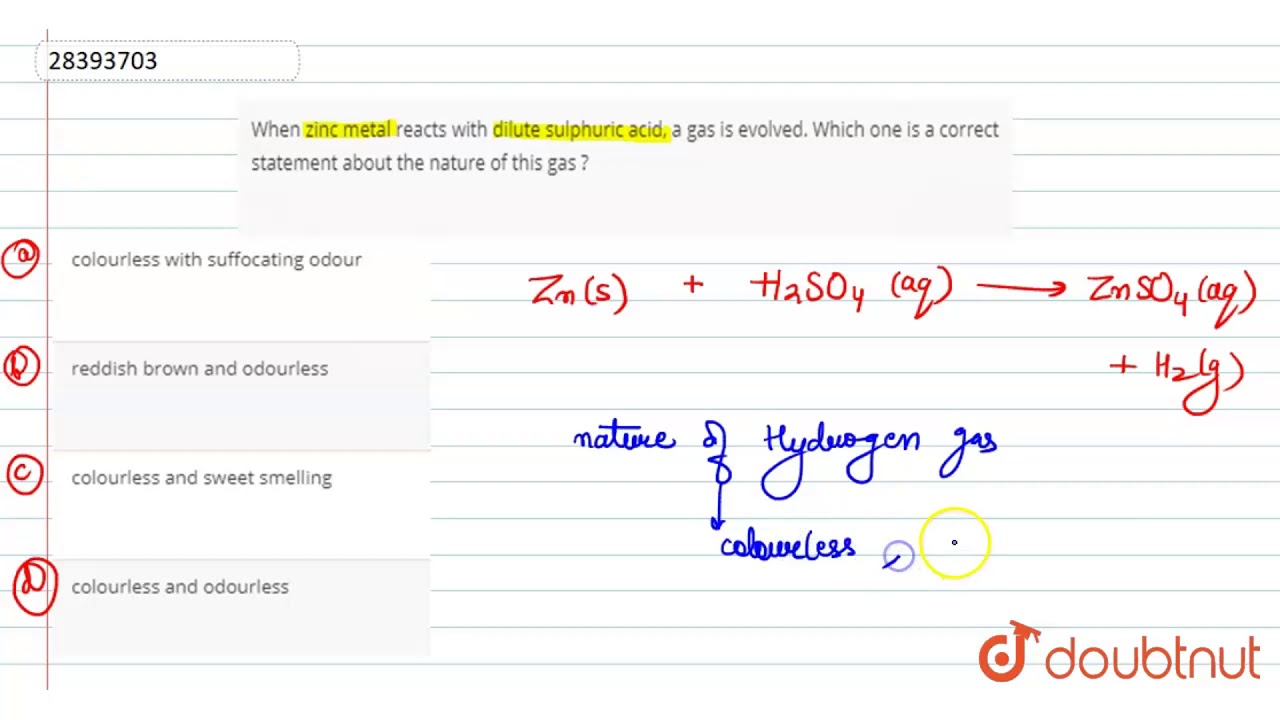
When Zinc Metal Reacts With Dilute Sulphuric Acid A Gas Is Evolved Which One Is A Correct Youtube
Solved Zinc Metal Reacts With Excess Hydrochloric Acid To Chegg Com
Solved 5 Zinc Metal Reacts With Hydrochloric Acid To Chegg Com
Solved Zinc Metal Reacts With Hydrochloric According To The Balanced Equation Quad Mathrm Zn S 2 Operatorname Hcl A Q Longrightarrow Mathrm Zncl
Solved 1 Zinc Metal Reacts With Hydrochloric Acid According Chegg Com
Answered 2 Aluminum Metal Reacts With Zinc Bartleby
Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com
What Would Be The Reaction Of Naoh With Zinc Metal Quora
Solved Consider This Reaction Zinc Metal Reacts With Chegg Com
Zinc Reacts With Dilute Hydrochloric Acid To Produce Hydrogen Gas A Write Down The Balanced Chemical Equation Of The Reaction Taking Place There B Which Type Of Reaction Is It Combination Reaction
Solved B A Sample Of Zinc Metal Reacts Completely With An Chegg Com
Determine How Many Grams Of Hydrogen Gas Are Produced From 27 2 G Of Hcl 4 Zinc Homeworklib
Please Help 2 When Zinc Metal Reacts With Copper Ii Nitrate Zinc Ii Nitrate And Copper Metal Are Homeworklib
Solved Zinc Metal Can Be Reacted With Sulfuric Acid To Produce Hydrogen Gas According T0 The Balanced Equation Zn S Hsqav Znsoa Eq Hx G The Balanced Redox Equation For This Reaction Is Zn S 2h Aq
10 Zinc Metal Reacts With Hydrochloric Acid To Form Zinc Chloride And Hydrogen Gas A Write Homeworklib
Comments
Post a Comment